
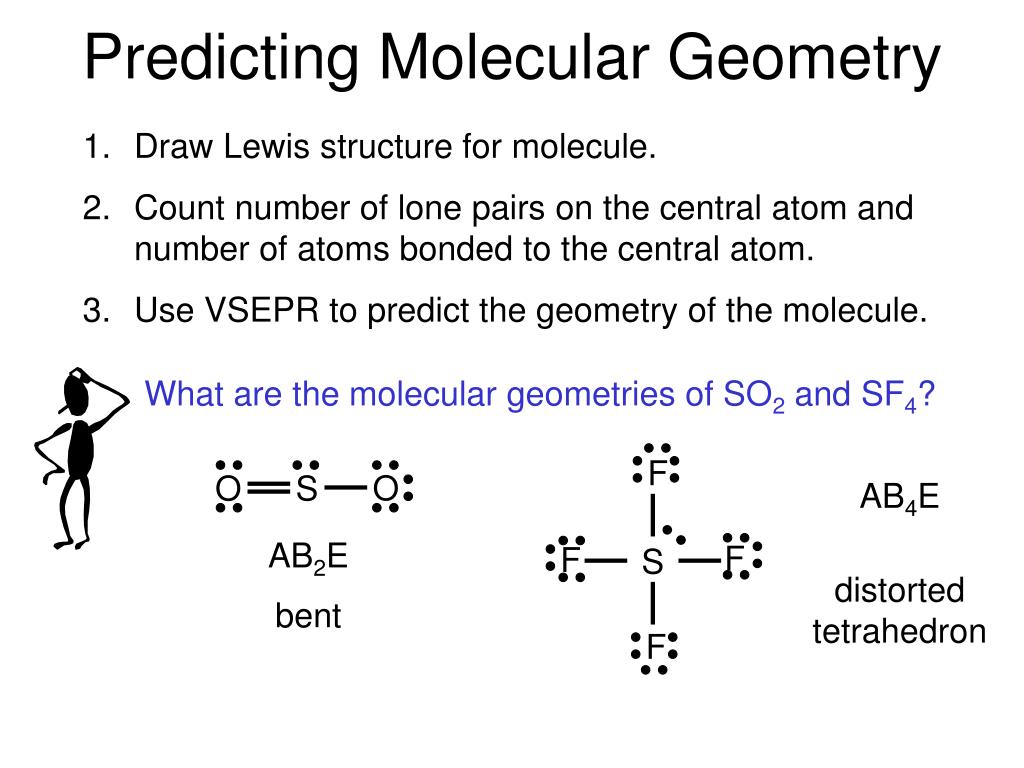
As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Remaining electrons in the p-orbitals in the Oxygen atom form pi bonds.Īs sp orbitals are hybridized to form the bonds, CO2 has an sp hybridization. These two hybridized orbitals overlap with the two p-orbitals of the Oxygen atom that results in the formation of sigma bonds. In contrast, the Oxygen atom hybridizes to form three sp2 hybrid orbitals.
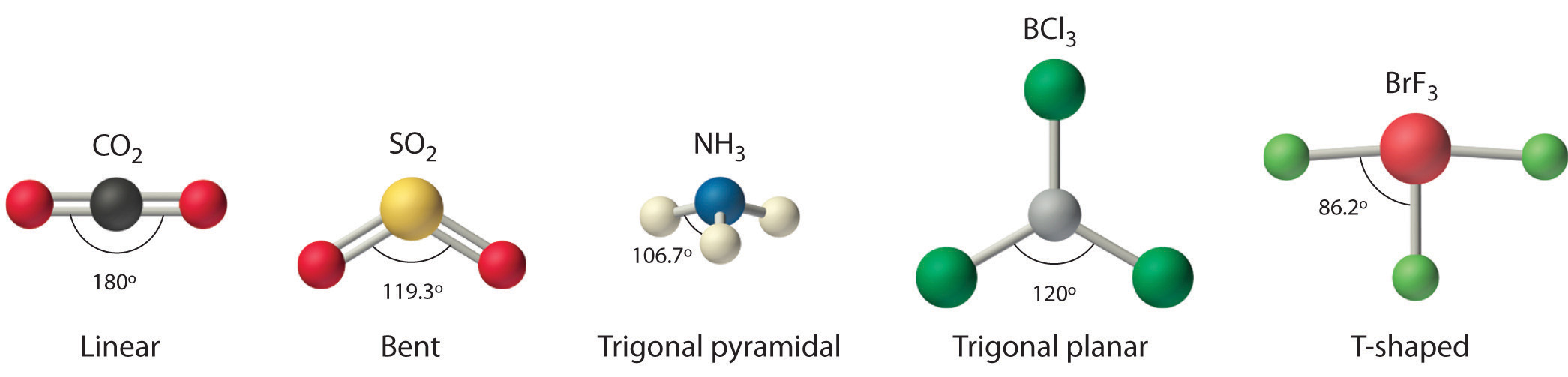
Here the 2s orbitals and one of the p-orbitals will hybridize to form 2 sp orbitals. In its excited state, the atom’s electronic configuration becomes 1s2 2s1 2p3, so now every p-orbital of the atoms has one electron each. Total number of valence electrons in the molecule = 16 Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2).To know the bond formation and the arrangement, let’s go through the valence electrons of all the atoms in the molecule. Two Oxygen atoms are located on the terminals where both these atoms share electrons and form bonds with the central Carbon atom. The molecular geometry of sulfur dioxide is a bent shape. Both the sulfur and oxygen atoms have six valence electrons. In CO2, the Carbon atom is in the central position as it is the least electronegative atom in the molecule. The SO2 Lewis structure would consist of two oxygen (O) atoms and one sulfur atom. Now that you know how the Lewis structure is drawn and its uses let us quickly look at the CO2 Lewis structure. Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. An explanation of the molecular geometry for the ClO2 (Chlorine dioxide) including a description of the ClO2 bond angles. Drawing lines represent the bonds formed in the molecule. These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure.
Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. This structure helps in knowing the arrangement of electrons in the molecules and the shape of the molecule. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. If these are all bond pairs the molecular geometry is tetrahedral (e.g. Name of molecule Carbon Dioxide ( CO2) No of Valence Electrons in the molecule 16 Hybridization of CO2 sp hybridization Bond Angles 180 degrees Molecular Geometry of CO2 Linear CO2 Lewis Structure For example four electron pairs are distributed in a tetrahedral shape.


 0 kommentar(er)
0 kommentar(er)
